Specimen Type: Serum
Turnaround Time: 2-3+ weeks
Test Marker: 1
Excess Homocysteine: A Key Factor in Cardiovascular Disease, Psychiatric Disorders, Cancer, Hip Fractures, and Alzheimer’s Disease
Homocysteine, a non-protein sulfur amino acid, is an intermediate product of the methionine cycle, also called the transsulfuration methylation cycle (Figure 1) that closely links sulfur amino acid metabolism with key methylation reactions. Homocysteine is formed from the catabolism of S-adenosylmethionine or by the conversion of toxic homocysteine thiolactone to homocysteine by paraoxonase 1 (PON1), the same enzyme that detoxifies organophosphate pesticides. The clearance of homocysteine requires the presence of methyltetrahydrofolate, methylcobalamin, betaine, or pyridoxal-5-phosphate. Methylcobalamin is such a powerful methylating co-enzyme that it can methylate homocysteine directly to methionine without an associated enzyme.
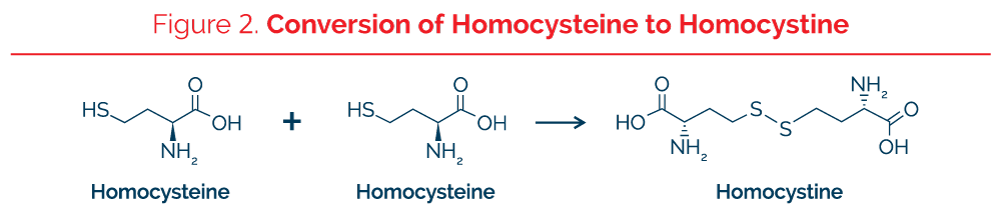
Kilmer McCully was one of the first investigators to develop an interest in the toxic effects of homocysteine as a major risk factor in atherosclerosis. After investigating cases of classic homocystinuria in patients who had abnormally increased plasma total homocysteine and severe atherosclerosis even as a teenager, McCully began to investigate moderate elevations of homocysteine as a cause of atherosclerosis in a large section of the entire population. Individuals with the severe genetic deficiency of the enzyme cystathionine b-synthase had concentrations of total plasma homocysteine as high as 300 mmol/L. High homocysteine is also associated with other cardiovascular risk factors such as insulin resistance, metabolic syndrome, and type 2 diabetes mellitus. The optimum plasma homocysteine appears to be 6-7 micromoles/liter. The amino acid homocysteine has a sulfhydryl group that is hydrogenated (SH) while homocysteine in the oxidized state is bonded to another sulfhydryl group, either to another homocysteine in which the molecule is called homocystine or oxidized homocysteine or to the sulfhydryl group of another different protein or peptide (Figure 2). In the test performed at The Great Plains Laboratory, all forms of the molecule are converted to the reduced form with a free –SH group prior to quantitation.
Metabolism of Homocysteine
While homocysteine is an amino acid, it is not one of the 20 amino acids found in proteins. It is most commonly formed in the pathway in which methionine is converted to S-adenosylmethionine (S-ame) and then S-adenosyl-homocysteine, and finally homocysteine. Homocysteine then can be metabolized predominantly by 3 different metabolic pathways. Two of those enzymatic pathways convert homocysteine back to methionine while the third enzymatic pathway converts homocysteine and serine to the amino acid cystathionine using the enzyme cystathionine beta-synthase (CBS). Two different enzymes can remethylate homocysteine back to methionine: methionine synthase (MTR) which requires N-5 methyltetrahydrofolate (CH3-THF) as a cofactor and betaine-homocysteine methyltransferase (BHMT), which transfers a methyl group from trimethylglycine (TMG) (also termed betaine) to homocysteine. In addition, homocysteine can be methylated directly by the coenzyme methylcobalamin without the use of an enzyme. Thus, methylcobalamin is a very useful nutritional supplement to reduce homocysteine even when a person has unfavorable genetic SNPs in Figure 1.
Cystathionine, the product of the CBS enzyme, is then broken down to form 2-oxobutyrate, cysteine, and ammonia and then 2-hydroxybutyric acid. Thus, 2-hydroxybutyric acid measured in the organic acid test is an indirect measurement of the metabolite flow proceeding through the CBS enzyme. Although CBS activity produces ammonia, high orotic acid, an indicator of increased ammonia, is not often elevated in people with high 2-hydroxybutyric acid, indicating this pathway is not a major source of ammonia.
It is thought that homocysteine may actually be the amino acid that is preferably selected by transfer-RNA to form homocysteine-transfer RNA in eukaryotic organisms but then is methylated to form methionine-transfer RNA. Homocysteine thiolactone is formed as a breakdown product if there are inadequate methylation factors available and then is converted to homocysteine by the enzyme paraoxonase 1 (PON1), the same enzyme that breaks down organophosphosphate pesticides. Since homocysteine thiolactone is toxic, high exposures to organophosphate pesticides might lead to inhibition of the breakdown of homocysteine thiolactone, a suspected carcinogen.
Factors that Elevate Homocysteine
Factors that increase homocysteine include severe or mild genetic deficiencies of enzymes in the transulfuration methylation cycle such as MTHFR, CBS, BHMT and others, a diet high in methionine, or deficiencies of folate, vitamin B12, vitamin B6, or betaine. Other factors that elevate homocysteine include megadoses of niacin, nitrous oxide anesthesia, and excessive copper.
Factors that Decrease Homocysteine
Homocysteine is decreased by a diet high in folate compounds, vitamin B12, methylcobalamin, betaine or supplements containing these vitamins. In addition, exposure to many toxic chemicals can upregulate CBS leading to increased conversion of homocysteine to cystathionine with a concomitant decrease of homocysteine. Conversely, removal of toxic chemicals may result in increased serum homocysteine.
Homocysteine and Cancer
In folate deficiency, uracil replaces thymine in DNA, inducing DNA strand breaks and hypomethylation within the hepatic p53 tumor suppressor gene, possibly increasing susceptibility to cancer. Studies showed that patients with ovarian, pancreatic, colorectal, head and neck squamous cell carcinomas and acute lymphoblastic leukemia had higher homocysteine levels. In addition, there was an increase in homocysteine levels in rapid proliferating tumor cell lines and plasma homocysteine levels declined when tumor cells started dying.
Homocysteine and Psychiatric Disorders
In a cross-sectional, prospective study involving data from 521 subjects over the age of 65 who were not depressed at baseline, who were followed for a period of two to three years, low serum levels of folate and vitamin B12, and high plasma levels of homocysteine were found to be associated with a higher risk of incident depression. There are several mechanisms linking elevated homocysteine to biological underpinnings of psychiatric disorders. It has been found that homocysteine interacts with NMDA receptors, initiates oxidative stress, induces apoptosis, triggers mitochondrial dysfunction, and leads to vascular damage. Elevated homocysteine levels might also contribute to cognitive impairment that is widely observed among patients with affective disorders and schizophrenia. Supplementation of B vitamins and folic acid has been proven to be effective in lowering homocysteine levels. L-methylfolate at doses between 7.5 to 15 mg per day plus antidepressants at treatment onset was more effective than antidepressants alone in improving depressive symptoms. In addition, symptoms were measured by clinical depression evaluation scores within 60 days of adding L-methylfolate to antidepressant therapy, which showed major symptomatic improvement more rapidly than SSRI/SNRI monotherapy, and it was better tolerated.
Homocysteine and Hip Fracture
Men and women in the highest quartile of serum homocysteine had a greater risk of hip fracture than those in the lowest quartile – the risk was almost four times as high for men and 1.9 times as high for women. It has been proposed that homocysteine interferes with the formation of cross-links in collagen, leading to osteoporosis. The association between homocysteine levels and the risk of fracture appeared to be independent of bone mineral density and other potential risk factors for fracture.
Utilizing the Homocysteine Marker for Optimum Health with Vitamin Therapy
In many cases, homocysteine can serve as a very useful marker for the overall efficacy of the transulfuration methylation cycle, as in the reported case of very high baseline homocysteine (52.9 micromole/liter) which was treated with injections of hydroxycobalamin. Upon re-testing, the patient’s homocysteine had dropped to 16.8 micromoles/liter, a 68% decrease.
A typical dose of vitamins commonly recommended for adults with elevated homocysteine is 2000 mcg methylcobalamin, 2000 mcg N5-methyltetrahydrofolate, and 50 mg of vitamin B6. After one week on this regimen, another blood sample is taken and homocysteine is measured again. If the repeat value for homocysteine is greater than 7.0, the doses of the vitamins are doubled and the test is repeated a week later. In some cases, 5-methyltetahydrofolate may have to be given at 5, 10, or even 15 mg per day as a pharmaceutical. If the homocysteine value is still high, the physician may want to check for excessive copper, reduce methionine in the diet, explore the possibility of the autoimmune disease pernicious anemia, or add another methylation cycle nutrient like betaine at doses of 250-500 mg daily. Since abnormally elevated homocysteine is commonly due to single nucleotide polymorphisms or (SNPs), it is likely that the patient will need to be on a lifetime regimen of the same supplements that brought levels into the optimum range.
When Homocysteine is Not Reliable as a Transulfuration Methylation Cycle Marker
One of the factors that can markedly alter homocysteine and make the homocysteine marker unreliable as an overall measure of the transsulfuration methylation cycle is an excess of cystathionine beta-synthase. One of the conditions in which this is prevalent is Down’s syndrome in which there is an excess of CBS because the CBS enzyme is on chromosome 21. Since Down’s syndrome is caused by having three copies of chromosome 21 instead of two copies, individuals with Down’s syndrome commonly have an overproduction of CBS which reduces homocysteine. At the same time, there is so much homocysteine channeled into the CBS reaction that production of methionine may be limited so that there is an acute need to increase the coenzymes methyltetrahydrofolate and methylcobalamin and the other methyl donor betaine.
CBS is also overproduced due to toxic exposures to a wide variety of chemicals so that the body can increase more glutathione from homocysteine to detoxify toxic chemicals. Thus, a person with severe toxic exposures might have a low homocysteine value rather than a high one. Low homocysteine values in autism may be associated with severe toxic chemical exposures, which have been confirmed in many cases of autism. In cases of toxicity, the baseline homocysteine may be below 7.0 due to the upregulation of CBS to increase glutathione. Peripheral neuropathy commonly caused by toxic chemical exposure is associated with low serum homocysteine. In cases of toxicity, serum homocysteine may increase after detoxification.
Organic acids testing is very useful in the above cases. Markers for excessive CBS activity (2-hydroxybutyric acid), toxicity (succinic acid), and glutathione deficiency (elevated pyroglutamic acid) are all useful markers to decipher the transulfuration methylation pathway. 2-Hydroxybutyric acid is a downstream product of CBS activity (Figure 1). In Down’s syndrome, 2-hydroxybutyric acid in urine will usually be high while plasma homocysteine is low. The same phenomenon of high 2-hydroxybutyric acid and low plasma homocysteine occurs with toxicity. In toxic exposure cases, succinic acid will often be high due to toxic inhibitory effects on succinic acid dehydrogenase in the Krebs cycle, while the glutathione deficiency marker pyroglutamic acid will be high, indicating glutathione deficiency.
References
Yes¸Im Özkan, Sevgi Yardim-Akaydin, Hikmet Firat, Emel Çalis¸Kan-Can, Sadik Ardiç and Bolkan S¸Ims¸Ek. Usefulness of Homocysteine as a Cancer Marker: Total Thiol Compounds and Folate Levels in Untreated Lung Cancer Patients ANTICANCER RESEARCH 27: 1185-1190 (2007)
Wu LL Wu JT Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta 2002 Aug;322(1-2):21-8.
Giuseppe Salemi, Maria Concetta Gueli, Francesco Vitale, Floriana Battaglieri, Egidio Guglielmini, Paolo Ragonese, Angela Trentacosti, Maria Fatima Massenti, Giovanni Savettieri, Antonino Bono. Blood lipids, homocysteine, stress factors, and vitamins in clinically stable multiple sclerosis patients. Lipids in Health and Disease 2010, 9:19
Tapan K. Basu*, Neelam Makhani and Gary Sedgwick Niacin (nicotinic acid) in non-physiological doses causes hyperhomocysteineaemia in Sprague–Dawley rats British Journal of Nutrition (2002), 87, 115–119
Melvin R Hayden and Suresh C Tyagi. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutrition Journal 2004, 3:4
Y. M. Smulders, D. E. C. Smith, R. M.Kok, 2 T. Teerlink, D. W. Swinkels, C. D. A. Stehouwer and C. Jakobs. Cellular folate vitamer distribution during and after correction of vitamin B12 deficiency: a case for the methylfolate trap. British Journal of Haematology, 132, 623–629
Lawrence D. Ginsberg; Alondra Y. Oubre D, and Yahya A. Daoud. L-methylfolate Plus SSRI or SNRI from Treatment Initiation Compared to SSRI or SNRI Monotherapy in a Major Depressive Episode. Innov Clin Neurosci. 2011;8(1):19–28.
Mohammad A. Mansoor, Claes Bergmark, Steve J. Haswell, Ian F. Savage, Peter H. Evans, Rolf K. Berge, Asbjørn M. Svardal and Ole Kristensen. Correlation between Plasma Total Homocysteine and Copper in Patients with Peripheral Vascular Disease. Clinical Chemistry 46:385–391 (2000)
Uffe Ravnskov, Michel de Lorgeril, David M Diamond, Rokuro Hama, Tomohito Hamazaki, Björn Hammarskjöld, Niamh Hynes, Malcolm Kendrick, Peter H Langsjoen, Luca Mascitelli, Kilmer S McCully, Harumi Okuyama, Paul J Rosch, Tore Schersten, Sherif Sultan & Ralf Sundberg (2018): LDL-C Does Not Cause Cardiovascular Disease: a comprehensive review of current literature, Expert Review of Clinical Pharmacology, DOI: 10.1080/17512433.2018.1519391
Celia M. Antonio, Marta C. Nunes, Helga Refsum and Abraham K. Abraham. A novel pathway for the conversion of homocysteine to methionine in eukaryotes. Biochem. J. (1997) 328, 165-170
Jia-Min Zhuo and Domenico Pratico Normalization of hyperhomocysteinemia improves cognitive deficits and ameliorates brain amyloidosis of a transgenic mouse model of Alzheimer’s disease. FASEB J. 24, 000–000 (2010)
McLean, RR; Jacques, PF; Selhub, J; Tucker, KL; Samelson, EJ; Broe, KE; Hannan, MT; Cupples, LA; Kiel, DP (May 13, 2004). “Homocysteine as a predictive factor for hip fracture in older persons”. The New England Journal of Medicine. 350 (20): 2042–9. doi:10.1056/NEJMoa032739. PMID 15141042.